Voluntary out-of-body experience: an fMRI study
- School of Psychology, University of Ottawa, Ottawa, ON, Canada
Introduction
The experience of one’s body is a central process to
allow us to interact with the outside world. Body experience is based on
the integration of visual, vestibular, and somatosensory information (Giummarra et al., 2008; Berlucchi and Aglioti, 2010; de Vignemont, 2011; Blanke, 2012; Moseley et al., 2012).
This information allows the tracking of the body in space and in
relation with other objects and beings in our environment. Tracking of
our body in turn, guides our movements (Goodale et al., 2008).
The conscious experience of our body is generally congruent across
sensory modalities so that, what we see of our body is also what we feel
from somatosensory and vestibular sensations (Tsakiris, 2010).
The sensations and percept associated with our body in movement can
also be elicited in our imagination albeit most of the time in an
attenuated form. Motor imagery corresponds to the cognitive version of
motor actions without actual motor movements (Guillot et al., 2012; Moran et al., 2012).
This motor “imagery” encompass visual components when we imagine
movements as we would see them from our own perspective or from a
third-person perspective (imagine someone else moving – or imagine
ourselves moving but from a third-person perspective) and proprioceptive
and vestibular components often referred to kinesthetic “imagery” (Guillot et al., 2009,
p. 698). Motor imagery is intertwined within the brain’s preparatory
processes preceding action and, up to a certain point, the brain’s
processes subserving actual movement (Guillot and Collet, 2005).
The strongest support for this view has come from functional imaging
that demonstrated strong but incomplete overlap between imagery, action
preparation, and action (Porro et al., 1996; Guillot et al., 2008, 2009; Szameitat et al., 2012a,b).
These studies show that motor imagery is dependent both on brain
regions associated with the performance of motor action but also on the
somatosensory brain regions associated with body perception. Voluntary
and involuntary motor imagery is also present in amputated individuals
with an associated phantom limb often together with somatosensory
perception (Melzack, 1989, p. 657; Ramachandran and Hirstein, 1998,
p. 493). Some amputees can also train themselves to experience an
anatomically impossible movement with their phantom limb suggesting the
plasticity of sensorimotor systems (Moseley and Brugger, 2009, p. 1069).
The multi-component nature of body representation is also revealed in perceptual illusions such as the rubber hand illusion (Botvinick and Cohen, 1998).
In the rubber hand illusion, the vision-based belief that the rubber
hand is not part of the participant’s body is countered by the
simultaneous touching of the rubber hand and the real hand and leads to a
shift in the attribution of the localization of sensory stimulation
from the real hand to the rubber hand (Hohwy and Paton, 2010).
During the process of establishing the illusion, from completely
separate to unity with the rubber hand, several intermediate illusory
experiences can take place (Valenzuela Moguillansky et al., 2013,
p. 1001). In one experiment using a moveable hand model, conditions
could be manipulated so that participants reported a dissociation of the
sense of ownership (impression that the fake hand is their own) or the
sense of agency (impression that participants controlled the movements
of the fake hand) (Kalckert and Ehrsson, 2012).
Mismatch between the observed position of the hand model and the sensed
position of the real hand reduced sense of ownership but did not
disrupt the impression of agency. Conversely, passive movement reduced
agency but left ownership intact (Kalckert and Ehrsson, 2012). These observations suggest that agency and ownership may depend on different but overlapping brain networks (Jackson et al., 2006,
p. 703). Another experiment demonstrated that concurrent limb and
full-body orientation illusions elicited by virtual reality visual
displacement were undissociated and not dependent on action (Olive and Berthoz, 2012, p. 1050).
During these illusions, the participants do not doubt that the shifted body perception is illusory (Blanke and Metzinger, 2009). In contrast, shifted body perception of neurological origin (Blanke and Mohr, 2005) or pharmacologically induced (Morgan et al., 2011; Wilkins et al., 2011)
can lead to ambiguous embodiment whereas people report that the
illusory body or body part is more realistic or corresponds to a
“double” of their body. In the descriptions below, the “double” refers
to the illusory body (or parts thereof). There seems to be a general
consensus in adopting the classification proposed by Brugger to describe
these illusions (Brugger and Regard, 1997).
Autoscopic hallucination is a visual hallucination of the upper part of
a double of the body. Heautoscopy is a visual and somesthetic
hallucination. The double, which appears as through a veil, can mirror
the person’s movements. Heautoscopy hallucination is also accompanied by
a vague feeling of detachment and depersonalization. The double is felt
vaguely as another self. Feeling of a presence is a mostly somesthetic
hallucination that a double is present usually close by or even touching
but not seen. Feeling of a presence is also called sensed-presence
experience when the presence is identified as another person (Cheyne and Girard, 2007,
p. 1065). Out-of-body experience is a visual and somesthetic experience
in which the double is seen from a different perspective, often
motionless. Because the body in this experience is “seen” from a
third-person perspective (i.e., from above), the body seen is illusory
even if it is congruent with the body’s position during the illusion
(e.g., lying down). The experience is accompanied by a profound feeling
of being outside of the body and with feelings of meaningfulness of the
experience.
Three studies of self-reported anomalous body experiences in unremarkable normal people (Braithwaite et al., 2011, p. 876; Braithwaite et al., 2011, p. 1063; Braithwaite et al., 2013,
p. 1064). In the first one, it was noted that most instances of
spontaneous anomalous body experiences occurred during a relaxed or
borderline sleeping state and one-third reported (seeing) their body
from a different perspective while the rest reported a visual or
somatosensory shift in perspective. The participants who reported
out-of-body experience also self-reported more perceptual anomalies (Braithwaite et al., 2011,
p. 876). In two subsequent experiments, participants self-reporting
anomalous body experiences (mostly of visual nature) were more likely to
respond strongly to aversive visual patterns suggesting that the visual
system of the participants are somehow different, at least functionally
(Braithwaite et al., 2013, p. 1064; Braithwaite et al., 2013,
p. 1063). The authors also derived the hypothesis that these anomalous
body experiences depended on temporal lobe anomalies as measured by
perceptual tasks and questionnaires (Braithwaite et al., 2011, p. 876).
There also have been imaging enquiries into the brain
areas involved in body representation illusions in neurologically
intact participants (Blanke, 2012).
Brain imaging studies have suggested that activity in sensory
integration areas such as the intraparietal sulcus and the ventral
premotor cortex are associated with the establishment of the rubber hand
illusion (Ehrsson et al., 2004, 2005, 2007; Tsakiris et al., 2007).
One experiment has used repeated transcranial magnetic stimulation to
gain information on the brain areas involved in the rubber hand illusion
(Tsakiris et al., 2008).
They found that, when the activity of the temporal parietal junction
(TPJ) was perturbed by repeated transcranial magnetic stimulation, the
processing of body representation mental imagery was impaired. However,
in another transcranial magnetic stimulation study, mental rotation of
letter stimuli was not affected suggesting a specific effect for body
representation (Blanke et al., 2005).
Another experiment showed that, the temporal parietal junction, which
is involved in self processing and multisensory integration of
body-related information; and the extrastriate body area (EBA), which
responds selectively to human bodies and body parts mental imagery is
performed with mentally embodied (EBA) or disembodied (TPJ) self
location (Arzy et al., 2006).
The more intense hallucinations or illusions are usually associated
with brain lesions, abnormal brain function such as epilepsy, major
psychiatric syndromes, dissociative drugs such as ketamine, or in
micro-gravity conditions (Kornilova, 1997).
The study of the lesioned or abnormal brain areas is
often used to gain insight into the brain areas involved in normal body
representation phenomena. However, there is also anecdotal evidence that
these intense hallucinations can occur in non-neurological cases but
they have a low occurrence and, apart from micro-gravity illusions, are
unpredictable. In the present report, we used functional MRI to examine
an otherwise “normal,” healthy individual that reported the ability to,
at will, vividly experience her body moving outside her physical body
while lying down at rest. The subjective description of the participant
led us to use the term extra-corporeal experience (ECE) throughout this
manuscript to underline the difference between the phenomenon studied
here and the more common definition of out-of-body experiences. We
included a number of guided imagery tasks to specify the ECE-related
brain activity. One control task was motor imagery for a different
movement (jumping jacks). A second control condition was alternating
between actual finger movements and motor imagery of the same movement.
Finally, we were interested in determining if there was a difference
between imagining herself performing the ECE (but not experiencing the
ECE) differed from the imagining of another person performing the same
ECE movement.
Materials and Methods
Participant
The participant was a right-handed woman, age 24, who
was a psychology graduate student at the time of testing. She signed an
informed consent approved by the University of Ottawa Research Ethics
Board. The participant was in an undergraduate class that presented data
on body representation hallucinations in patients that report
experiences of their body outside their physical body (Blanke and Arzy, 2005).
The participant spontaneously reported after class that she could have a
similar “out of body” experience. She appeared surprised that not
everyone could experience this. The participant described her experience
as one she began performing as a child when bored with “sleep time” at
preschool. She discovered she could elicit the experience of moving
above her body and used this as a distraction during the time kids were
asked to nap. She continued to perform this experience as she grew up
assuming, as mentioned, that “everyone could do it.” This was often done
before sleep onset as an aid to enter sleep. She described the
experience as variable depending on her frame of mind. She was able to
see herself rotating in the air above her body, lying flat, and rolling
along with the horizontal plane. She reported sometimes watching herself
move from above but remained aware of her unmoving “real” body. The
participant reported no particular emotions linked to the experience. As
an adult, the participant only infrequently “practiced” the experience;
the experience does not occur spontaneously but is induced wilfully.
The participant describes the experience in the following terms: “I feel
myself moving, or, more accurately, can make myself feel as if I am
moving. I know perfectly well that I am not actually moving. There is no
duality of body and mind when this happens, not really. In fact, I am
hyper-sensitive to my body at that point, because I am concentrating so
hard on the sensation of moving. I am the one moving – me – my body. For
example, if I ‘spin’ for long enough, I get dizzy. I do not see myself
above my body. Rather, my whole body has moved up. I feel it as being
above where I know it actually is. I usually also picture myself as
moving up in my mind’s eye, but the mind is not substantive. It does not
move unless the body does.”
Procedure
Four questionnaires were administered. The Pittsburgh Sleep Quality Index (Buysse et al., 1989)
was used to detect possible sleep disturbances because sleep onset
disturbances have been associated with altered somatosensory or
vestibular perceptions (Braithwaite et al., 2011).
In order to estimate visual and kinesthetic imagery, the participant
was asked to complete the 8-item Movement Imagery Questionnaire-Revised
(MIQ-R; Hall and Martin, 1997) and the 20-item Kinesthetic and Visual Imagery Questionnaire (KVIQ; Malouin et al., 2007). Finally, the PAS perceptual aberration scale (Arzy et al., 2007) was administered.
Data Acquisition
The experimenter provided instructions to the
participant through MRI earphones. The data was collected in one imaging
session during which time both anatomical and functional MR images were
obtained. All imaging was performed using a 1.5-T Siemens Magnetom
Symphony MRI scanner. The participant lay supine with her head secured
in a custom head holder. A conventional T1-weighted spin echo localizer
was acquired and used to prescribe a subsequent 3D FLASH (TR/TE 11.2/21
ms, flip angle 60°, field of view (FOV) 26 cm × 26 cm, 256 × 256 matrix,
slice thickness 1.5 mm) volume acquisition used for further structural
analyses. A T2 FLAIR scan was also performed and inspected by a
neuroradiologist following the scanning session to ensure that there was
no structural anomaly. Whole brain fMRI was performed using a
T2*-weighted echo planar pulse sequence (TR/TE 3000/40 ms, flip angle
90°, FOV 24 cm × 24 cm, 64 × 64 matrix, slice thickness 5 mm, 27 axial
slices, bandwidth 62.5 kHz).
Table 1
presents the order and characteristics of each run. The participant was
asked after the structural images were acquired if she believed she
would be able to “perform” her ECE: she reported being certain she
could. Functional imaging runs lasted 59 min in total with an additional
10 min consisting of instructions between runs. Six functional “runs”
in the scanner using a block design took place. Runs 1, 4, and 6
involved the participant going in and out of her ECE experience for 5
min at the researcher’s oral command of “start” and “stop.” She induced
the ECE to the researcher’s command of “start” and then was stopped
after 90 s with the word “stop.” This was repeated four times for Runs 1
and 6 and three times for Run 4. The participant was asked to perform
her ECE at the “start” prompt and to tap her finger when she felt
herself starting. Prior to imaging she had practiced this tapping at
home to ensure it would not interfere with her performance. She was
asked to tap her finger again if the ECE stopped before the researcher
said “stop.” As this was the case on two trials the blocks were adjusted
to maximize the data obtained and the image analysis included scans
from the ECE blocks and the rest blocks. If she concluded her ECE prior
to the experimenter stopping her she would again tap her finger (in
sight of the researchers). In Run 1, the ECE consisted of being above
her body and rocking from side-to-side. The participant reported having
trouble stopping the rocking movement. The participant also signaled if
the movement stopped during the run – the time the movement stopped and
re-started was recorded for subsequent analysis. In Run 4, the
participant was asked to perform an ECE (above her body and spinning
horizontally) and to tap her finger when she felt herself starting. The
participant reported difficulty starting the movement (the onset of each
sub-run was always delayed contrary to other runs – all timings delays
were accounted for in the data analysis). The participant reported that
the spinning movement was hard to stop for the rest period. Because the
participant in general does not like the spinning movement (she gets
dizzy), she switched to a “bobbing on the ocean” movement during Run 4
and informed the experimenter after the end of that run. In Run 6, the
ECE was the bobbing movement: the participant reported the sub runs as
being less “sharp.”
TABLE 1
The second, third, and fifth
runs were guided motor imagery. Run 2 included an experimenter
instructing with one word (either “someone” or “you”) every 30 s,
alternating while she visualized (but not experienced) herself actually moving as she did in the ECE or while she visualized
someone else doing the same movement. This was a 5-min task. The
informal comment from the participant was that she did not “feel herself
moving” when “visualizing” her experience during run 2. We were
interested in determining if there was a difference between imagining
herself performing the ECE (but not experiencing the ECE) differed from
the imaging of another person performing the same ECE movement. Run 3
included the same alternating block design whereby the participant
imagined herself performing jumping jacks or resting: this
was a control task to determine which structures were involved in
non-ECE motor imagery. The participant practiced the instructions for
Run 3 prior to starting the run to ensure that she was able to visualize
herself. From the participant’s comments, it was inferred that
visualizing herself doing jumping jacks did not involve the movement
sensations associated with her extra corporeal experience. Run 5
involved the participant moving her right hand fingers (one at a time) to her thumb at a frequency of 2 Hz and then visualizing
herself perform the same movement. Again, the participant did not
report a sensation of movement. This control task was added to determine
the brain areas involved in a simple motor action and its imagined
version. Again, each block was 30 s and the Run was 5 min. Our
conversations with the participant suggested that her extra corporeal
experience involved the sensation of movement while other imagery tasks
she performed did not involve this sensation.
Image Post-Processing
The functional images were reconstructed and whole
brain images were realigned to correct for motion by employing the
procedure of Friston et al. (1995),
using Statistical Parametric Mapping (SPM8) software. The motion
correction did not exceed 1 mm. Images were spatially normalized to
match the echo planar imaging (EPI) template provided in SPM8 with 2 mm ×
2 mm × 2 mm voxel sizes. Images were then smoothed with a 10 mm
full-width at half-maximum Gaussian filter.
Statistical Analyses
A fixed effects analysis was performed with data from
each Run separately. The blocks of ECE were compared with the rest
blocks from the same Run. The Runs with motor imagery and/or
visualizations were analyzed by contrasting the two types of blocks, for
example in Run 3 scans from the rest blocks were subtracted from the
visualization of jumping jack blocks (Jumping Jacks minus Rest).
Results
Questionnaires
The MIQ-R results indicated that the participant had
kinesthetic imagery comparable to that observed in competitive sport
athletes (M = 5.5) but higher visual imagery (M = 7) (Roberts et al., 2008).
In the KVIQ, the participant scored an average of 4.1 on the visual
imagery scale (comparable to healthy but older controls) and 4.3 on the
kinesthetic imagery scale, which is higher than the same controls. The
Pittsburgh Sleep Quality Index (PSQI = 5) was slightly higher than would
be expected in healthy participants (PSQI = 2.67): this was essentially
due to longer sleep latency (90 min). In the PAS perceptual aberration
scale, the participant responded “false” to most statements except for
the following items (her answers in italics): (T.12) Now and then, when I
look in the mirror, my face seems quite different than usual. (Only when contemplating my own mortality); (T.15) Sometimes when I look at things like tables and chairs, they seem strange. (Occasionally but voluntary. Sometimes
late at night, I can play with perspective i.e., make things appear
closer/farther away. Also, sometimes, ordinary objects seem bizarre in
the sense that all existence is bizarre); (T.23) It has seemed at times as if my body was melting into my surroundings. (Always voluntary. I can make it feel like my body is going down into my bed); (T.31) Sometimes I feel like everything around me is tilting. (Almost always this is voluntary … usually when I am bored in class).
ECE Results
The participant reported being successful at
beginning and ending her ECE on demand of the experimenter. The
experience for Run 1 began immediately and she began to see herself
above her body rocking with her feet moving down and up as her head
moved up and down as in bobbing in ocean waves. The second ECE Run was
the most intense and involved the participant watching herself above her
own body, spinning along the horizontal axis. The final ECE involved
the participant spinning as in the second ECE.
Neural activation patterns for each of these ECE Runs
were analyzed separately with rest subtracted from the experience. Given
the lack of significant difference between the results of each of the
three Runs, all ECE Runs were combined into one analysis to increase
power and observe brain regions that were concomitantly activated for
each Run. Results are reported with a family wise error (FWE) very
stringent correction for multiple comparisons at 0.001. Results are
presented in Figure 1.
The most significantly and consistently activated areas during the ECE
compared to the non-ECE blocks were left lateralized in the
supplementary motor area (SMA) (x, y, z = −2, −18, 62, cluster 247, T = 6.66, p = 0.001), supramarginal gyrus/posterior superior temporal gyrus (x, y, z = −64, −46, 24, cluster 60, T = 6.04, p = 0.001), inferior temporal gyrus (x, y, z = −48, −54, −20, cluster 72, T = 5.89, p = 0.001), middle and superior orbital frontal gyri (x, y, z, = −26, 56, −10, T = 5.05, p = 0.001), and the cerebellum (x, y, z = −50, −48, −30, T = 5.76, p
= 0.001). The parietal and superior temporal activation taken together
correspond to the temporal parietal junction. There was significantly
less activation during the ECE blocks compared to non-ECE blocks (Figure
2) in bilateral posterior visual regions: the lingual gyrus (x, y, z = 14, −64, 4, cluster 19205, T = 13.23, p = 0.001) and the cuneus (x, y, z = 0, −92, 18, cluster 19205, T = 12.71, p = 0.001).
FIGURE 1
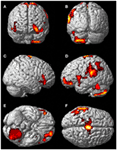 Figure 1. Rendered image of significantly activated
regions of the brain while the participant was having extra-corporeal
experiences. Most significantly activated regions are lateralized to the left side and include the supplementary motor area (F), the cerebellum (B,D,E), the supramarginal gyrus (D,F), the inferior temporal gyrus (B,D,F), the middle and superior orbitofrontal gyri (A,C,D,E). The p-value was set at 0.001 uncorrected for this image with the cluster threshold at 200 significant voxels.
Figure 1. Rendered image of significantly activated
regions of the brain while the participant was having extra-corporeal
experiences. Most significantly activated regions are lateralized to the left side and include the supplementary motor area (F), the cerebellum (B,D,E), the supramarginal gyrus (D,F), the inferior temporal gyrus (B,D,F), the middle and superior orbitofrontal gyri (A,C,D,E). The p-value was set at 0.001 uncorrected for this image with the cluster threshold at 200 significant voxels.
FIGURE 2
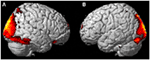 Figure 2. Areas of reduced activity during the ECEs compared to rest. The visual cortex is particularly impacted. (A) Representation of the right side; (B) activity on the left. The p-value for this image was set at 0.05 FWE corrected.
Figure 2. Areas of reduced activity during the ECEs compared to rest. The visual cortex is particularly impacted. (A) Representation of the right side; (B) activity on the left. The p-value for this image was set at 0.05 FWE corrected.Visualization Results
During imagining herself moving as she did in the
first ECE (Run 1), without inducing an ECE, controlling for multiple
comparisons at a p < 0.001, the participant activated more left cerebellum (x, y, z = −46, −48, −44, cluster 406, T = 5.66, p = 0.001) and bilateral lingual gyrus (x, y, z = −14, −62, 6, cluster 980, T = 5.00, p = 0.001; x, y, z = 6, −58, 8, cluster 790, T = 4.82, p = 0.001) than when imagining someone else moving in the same way (Figure 3).
Similarly, she showed significantly less activity during self-imagining
than imagining someone else in the bilateral superior orbital frontal
gyrus (x, y, z = −18, 66, −2, cluster 148, T = 4.40, p = 0.025; x, y, z = 14, 68, −2, cluster 146, T = 4.38, p = 0.026).
FIGURE 3
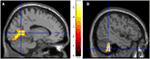 Figure 3. Results from visualizing herself doing the
same action she performed in the first ECE vs. visualizing another
person performing the same movement. (A) Bilateral lingual gyrus differences in activity and (B) the left cerebellar differences. The p-value for this image was set at 0.001 uncorrected.
Figure 3. Results from visualizing herself doing the
same action she performed in the first ECE vs. visualizing another
person performing the same movement. (A) Bilateral lingual gyrus differences in activity and (B) the left cerebellar differences. The p-value for this image was set at 0.001 uncorrected.
The second control task involved
the participant imagining herself performing jumping jacks and then not
imagining anything and just keeping her eyes closed waiting for the next
start cue for the jumping jacks. Results are presented in Figure 4. The imagining of herself performing the jumping jacks, controlling for multiple comparisons at p < 0.001, revealed significantly more activity in the posterior SMA (x, y, z = −2, −10, 60, cluster 1424, T = 7.95, p = 0.001), paracentral lobule (x, y, z = 0, −12, 68, cluster 1424, T = 6.72, p = 0.001), middle temporal gyrus (BA22) (x, y, z = 68, −48, 8, cluster 132, T = 5.72, p = 0.04), precentral gyrus (BA44) (x, y, z = −60, 6, 22, cluster 136, T = 5.11, p = 0.035), inferior parietal lobule (x, y, z = −40, −64, 58, cluster 265, T = 4.64, p = 0.001), and superior temporal gyrus (BA22) (x, y, z = 68, −34, 12, cluster 156, T = 4.78, p = 0.019). The TPJ activity was more bilateral than during the ECE runs (Figure 4). There was also less activity in bilateral cuneus (x, y, z = 6, −76, 4, cluster 22067, T = 10.16, p = 0.001) and bilateral superior orbital frontal gyrus (x, y, z = −28, 26, −28, cluster 617, T = 6.50, p = 0.001; x, y, z = 4, 48, −28, cluster 455, T = 5.69, p = 0.001) during the jumping jack imagery compared to rest.
FIGURE 4
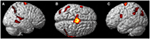 Figure 4. Results from visualizing herself performing jumping jacks compared to rest. (A) Right hemisphere; (B) dorsal view of the SMA activity; and (C) left hemisphere activation. The p-value for this image was set to 0.001 uncorrected with the cluster threshold at 100 significant voxels.
Figure 4. Results from visualizing herself performing jumping jacks compared to rest. (A) Right hemisphere; (B) dorsal view of the SMA activity; and (C) left hemisphere activation. The p-value for this image was set to 0.001 uncorrected with the cluster threshold at 100 significant voxels.
Another contrast of interest was
the actual movement of the fingers to the thumb compared with imagining
the same movement (Figure 5).
There was significantly more activation during the imagining vs. the
actual movement in several areas that were similarly (but not
identically) activated during the ECE. These included the bilateral
inferior frontal triangularis (x, y, z = 50, 40, −14, cluster 326, T = 5.27, p = 0.001; x, y, z = −42, 58, 0, cluster 1132, T = 5.18, p = 0.001), left middle temporal gyrus (x, y, z = −62, −58, −2, cluster 371, T = 6.31, p = 0.001), left cerebellum (x, y, z = −22, −88, −46, cluster 270, T = 5.97, p = 0.002), left superior parietal lobule (x, y, z = −36, −60, 50, cluster 581, T = 5.56, p = 0.001), and a more anterior part of the SMA (bilateral) (x, y, z = 0, 14, 58, cluster 711, T = 5.56, p = 0.001). Finally, there was significantly less activity during imagining than movement (Figure 6) in the left postcentral and precentral gyri (x, y, z = −32, −30, 70, cluster 1756, T = 12.85, p = 0.001; x, y, z = −36, −30, 62, cluster 1756, T = 12.05, p = 0.001, respectively), and right cerebellum (x, y, z = 10, −56, −22, cluster 997, T = 9.95, p = 0.001), areas similar to those activated during the jumping jack condition.
FIGURE 5
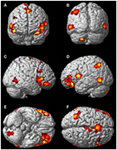 Figure 5. There was significantly more activation during the visualization of finger movement compared to the actual movement. Each letter represents a different view of the brain (A) anterior view, (B) posterior view, (C) right lateral view, (D) left lateral view, (E) ventral view, and (F) dorsal view. The p-value for this image was set to 0.001 uncorrected with the cluster threshold at 100 significant voxels.
Figure 5. There was significantly more activation during the visualization of finger movement compared to the actual movement. Each letter represents a different view of the brain (A) anterior view, (B) posterior view, (C) right lateral view, (D) left lateral view, (E) ventral view, and (F) dorsal view. The p-value for this image was set to 0.001 uncorrected with the cluster threshold at 100 significant voxels.
FIGURE 6
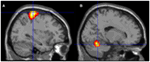 Figure 6. Motor areas significantly activated more
during movement of her fingers to thumb compared with visualizing the
same movement. (A) Representation of the left primary motor cortex; (B) representation of the right cerebellum. The p-value for this image was set to 0.001 uncorrected with the cluster threshold at 100 significant voxels.
Figure 6. Motor areas significantly activated more
during movement of her fingers to thumb compared with visualizing the
same movement. (A) Representation of the left primary motor cortex; (B) representation of the right cerebellum. The p-value for this image was set to 0.001 uncorrected with the cluster threshold at 100 significant voxels.Discussion
The present experiment examined functional brain
imaging patterns in a participant that reported being able, at will, to
produce somatosensory sensations that are experienced as her body moving
outside the boundaries of her physical body while remaining aware of
her unmoving physical body. It is interesting that the development of
the participant’s ability was associated with delayed sleep onset in
childhood (which persisted in adulthood) because the occurrence of
out-of-body experiences has been frequently associated with hypnagogic
phenomena (Cheyne et al., 1999; Terhune, 2009).
The reported experience is similar to what is defined by Brugger as an
out-of-body experience but without the feeling of being only outside of
her body and without any of the emotional content typically reported in
out-of-body experiences (Brugger and Regard, 1997).
The subjective description of the participant led us to use the term
ECE throughout this manuscript to underline the difference between the
phenomenon studied here and the more common definition of out-of-body
experiences. Also, because the ECE was private to the participant, we
have to rely on the participant’s descriptions to interpret the results.
With these caveats in mind, we find that the brain functional changes
associated with the reported ECE were different than those observed in
motor imagery. The results suggest that the ECE reported here represents
an unusual type of kinesthetic imagery that shares some features of
previously described out-of-body experiences and some features of more
typical motor imagery.
The ECE was reported as a mixture of visual imagery
and kinesthetic imagery but the kinesthetic component was prominent as
evidenced by the report of feeling dizzy when performing a rotational
movement. The prominence of kinesthetic experience over the visual
experience is consistent with a strong bilateral deactivation of the
lingual gyrus and cuneus encompassing the primary visual cortex.
Activations are mainly left-sided and involve the left SMA,
supramarginal and posterior superior temporal gyri (the last two overlap
with the temporal parietal junction, which has been associated with
out-of-body experiences). The cerebellum also shows strong activation
that is consistent with the participant’s report of the impression of
movement during the ECE. There are also left middle and superior orbital
frontal gyri activations, structures often associated with action
monitoring.
The TPJ activation that was observed during the ECE
is consistent with patient cases that report autoscopy and out-of-body
experiences when the functional integrity of that area is altered (Blanke et al., 2004; Blanke and Mohr, 2005; Blanke, 2012).
Studies of experimentally induced altered body imagery have
demonstrated that transcranial magnetic stimulation of the TPJ area can
interfere with the ability of healthy individuals to imagine themselves
in body orientations similar to out-of-body experiences (Blanke et al., 2005).
Electrical stimulation of the TPJ in epileptic patients also produces
various sensations associated with out-of-body experience (Blanke et al., 2002).
Interestingly, several of the active clusters found in the present
experiment during the ECE (left supramarginal gyrus, left inferior
temporal gyrus, left cerebellum) correspond closely to clusters with
mirror properties associated with action observation and execution that
were identified by a recent meta-analysis (Molenberghs et al., 2012).
The middle orbital frontal gyrus is a highly
multimodal area that has been associated with performance monitoring and
provides flexibility in response to selection based on ongoing feedback
(Elliott et al., 2000). The cluster that we observed in the left orbital frontal gyrus corresponds to cluster 6 of the K-6 solution described by (Kahnt et al., 2012) in their parcelation of the orbitofrontal cortex (Kahnt et al., 2012).
They reported functional connectivity with adjacent regions in the
lateral prefrontal cortex as well as regions in the inferior parietal
cortex and the lateral inferior temporal cortex; the latter two
structures correspond to activations we observed during the ECE.
We also instructed the participant to alternate
between visualizing herself performing her ECE and visualizing someone
else performing the same movement with the specific instruction that she
should not experience the ECE but only “see” it. The goal was to guide
the participant toward taking a first-person perspective of her own
experience and transposing it to a third-person perspective. The
first-person perspective was associated with a bilateral increase in the
lingual gyrus and another one in the left cerebellum: this may indicate
that imagining herself included both a visual component and possibly a
kinesthetic component (even following a specific instruction to avoid
this) that was absent when visualizing using the third-person view. The
self-visualization was accompanied by a reduction in orbitofrontal
activation that may indicate that visualizing herself was easier than
taking the third-person view and required less monitoring of activity. Jackson et al. (2006)
studied activations in participants observing hand or foot movements
seen either from a first-person perspective or a third-person
perspective. They found significantly more activity in the left
sensory-motor cortex for first-person, during observation alone, and in
the lingual gyrus for third-person perspective suggesting that
perspective taking is associated with a different pattern of activation (Jackson et al., 2006).
It is difficult to reconcile the higher lingual cortex activity
observed with our participant taking the first-person view and the
higher activity with the third-person perspective in Jackson et al. (2006).
However, in that study, participants were only shown pictures
corresponding to first- or third-person view of static limbs whereas our
participant was instructed to visualize a whole body movement. A
similar procedure contrasting first and third-person view was used in a
study in which participants viewed hand movements from the two
perspectives (Lorey et al., 2009)
and in a study where participants were instructed to imagine using a
tool presented to them on a picture or imagine someone else using the
same tool (Ruby and Decety, 2001).
Both these studies reported activation differences when contrasting
first- and third-person views. Our results obtained comparing first- and
third-person perspective for the ECE experience is similar in that
activation differences were observed between the two conditions when the
participant “only imagined” the ECE. The pattern of differences that we
observed was unsurprisingly quite different than in previous studies
likely owing to the task differences and the number of participants (Ruby and Decety, 2001; Lorey et al., 2009).
In the third condition, we examined the brain areas
involved in a whole body motor imagery to examine if the ECE was similar
to motor imagery in this participant. The first general observation is
that in this condition, activations tended to be bilateral as opposed to
mainly left-sided activations observed in the ECE. The second
observation is that the activations when the participant was told to
imagine doing jumping jacks were less extensive than for the ECE. They
included bilateral SMA extending into the paracentral lobule, bilateral
inferior parietal lobule, right middle and superior temporal gyri, and
left precentral gyrus. There was reduced activity in the cuneus
bilaterally and in the superior orbital frontal gyrus also bilaterally.
Activations of the SMA, inferior parietal lobule, and precentral gyrus
have been reported in two previous studies of kinesthetic imagery using
hand movements (Guillot et al., 2009; Szameitat et al., 2012b).
ECE and whole body motor imagery were both associated with a reduction
in cuneus activation (but less so for motor imagery) suggesting that
visual imagery was inhibited during both conditions. During motor
imagery, there was less activity in the superior orbital frontal cortex
whereas there was more activity in the middle and superior orbital
frontal cortex during ECE. This is suggestive of more motor monitoring
during ECE than motor imagery.
The last condition was an attempt to compare the
activations associated with actual hand movements to imagining the same
movement in this participant (Guillot et al., 2009; Szameitat et al., 2012b). In one of these studies, there were 13 participants selected on the basis of excellent motor imagery (Guillot et al., 2009) whereas the other included 21 unselected participants (Szameitat et al., 2012b).
The number of participants in both these studies achieved a greater
statistical power and reported many more activations than in the present
single-case study. The finger movements used in the Guillot et al.
study was a learned and practiced sequence, more complex than the one we
used, which could be considered more of an automatic nature. The
movement used in the Szameitat et al. study consisted of a simple wrist
movement timed with a tone. Although it is not clear how comparable
these studies are with the present observations, there are a number of
concordant findings. First, real and imagined movements produce
activations in the SMA. The activations reported by Szameitat et al. (2012a,b)
in the contrast imagery-rest include premotor areas in the precentral
gyrus, superior frontal gyrus, and bilateral inferior frontal gyri that
were also observed in the “jumping jacks” condition of our participant.
It has been shown that visual imagery is reliant on
the occipital lobe and the superior parietal lobule, as well as lateral
premotor cortex, while kinesthetic imagery is more associated with motor
areas and inferior parietal activity (Guillot et al., 2009,
p. 698). The ECE in the present study activated the left side of
several areas associated with kinesthetic imagery and was associated
with a strong deactivation of the visual cortex. This suggests that her
experience really was a novel one, with a strong kinesthetic component.
This was a healthy young woman with no brain abnormalities, thus
providing a window into the brain during non-pathological, self-elicited
ECE.
There are a number of limitations to the present
study. The first obvious one is that we relied on the participant’s
report of her experience. Given that the participant spontaneously
reported her experience assuming that it was a common occurrence and the
detailed (and unusual) description of how she developed this ability,
we are inclined to take her report at face value. The private nature of
imagery is common to most research in imagery (including other imagery
conditions in the present report) although a number of control measures
have been devised but they were not used here. One example of such
measures is the increase in heart rate and pulmonary ventilation during
imagined actions (Decety et al., 1993; Wuyam et al., 1995).
The description of the imagery tasks could have been more clearly
specified including the “jumping jacks” condition and the third-person
ECE task (Moran et al., 2012).
Statistical power was obviously limited in this single-case study,
which means that potentially several activations escaped detection.
Limited statistical power could also have prevented us from finding
activation differences when the participant performed “variations” of
her ECE experience (spinning vs. “bobbing on the ocean”).
This is the first study with a non-pathological
participant who is able to elicit an ECE upon demand. Clearly,
replication is required to ascertain if this pattern of activation is
similar in other people who can have self-initiated ECE. The existence
of such a case and its presentation raises the possibility that this
phenomenon may have a significant incidence but unreported because
people do not think this is exceptional. Alternatively, the ability
might be present in infancy but is lost without regular practice. This
would be reminiscent of the discovery and eventual study of synesthesia
that some researchers now hypothesized is more prevalent in young people
or can be developed (Deroy and Spence, 2013; Simner, 2013)

 Andra M. Smith
Andra M. Smith Claude Messier
Claude Messier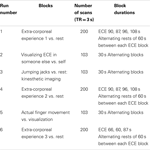
No comments:
Post a Comment